Algorithm for Optimized mRNA Design Improves Stability and Immunogenicity
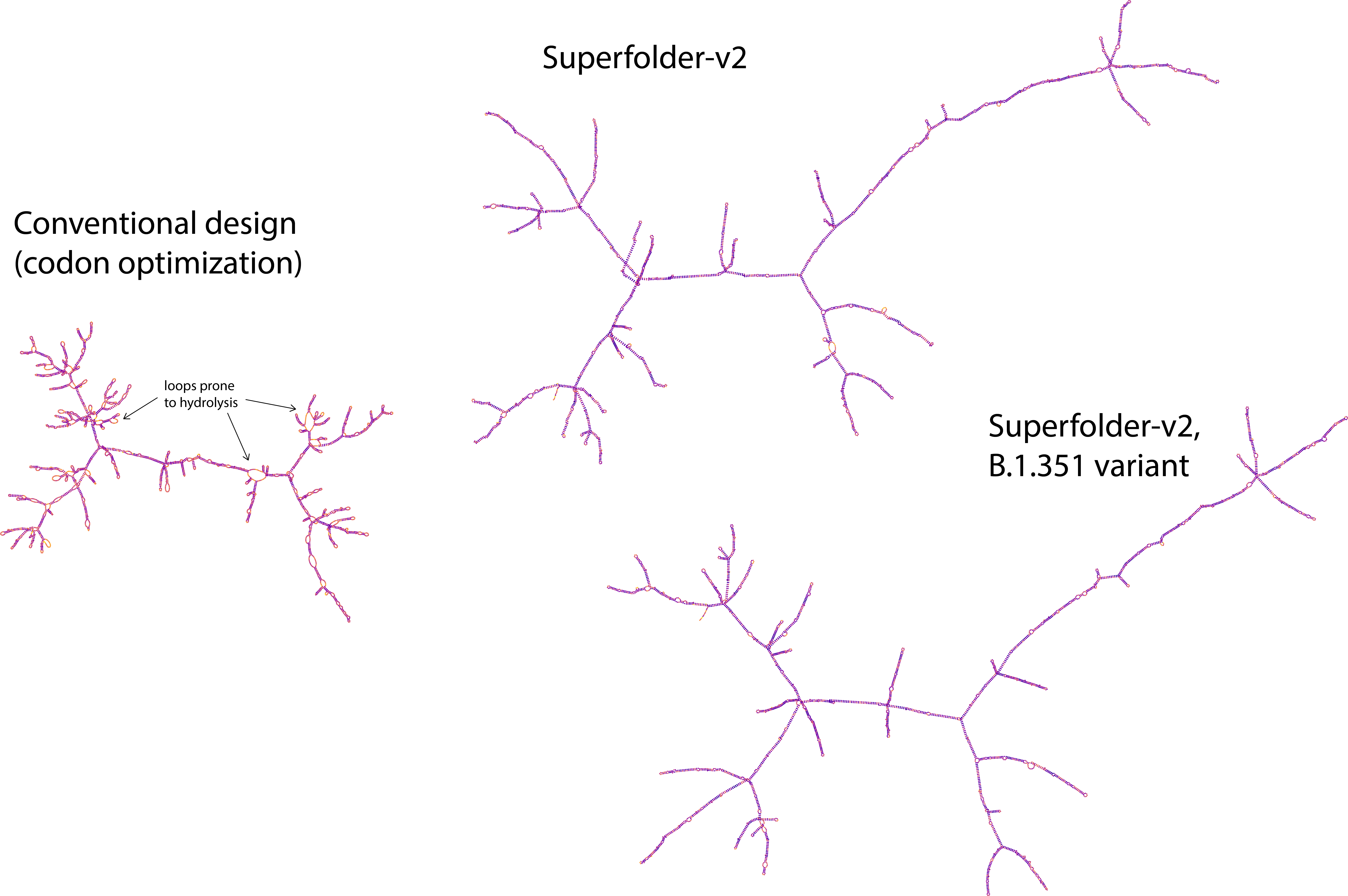
Messenger RNA (mRNA) vaccines are being used for COVID-19, but still suffer from the critical issue of mRNA instability and degradation, which is a major obstacle in the storage, distribution, and efficacy of the vaccine. Previous work showed that optimizing secondary structure stability lengthens mRNA half-life, which, together with optimal codons, increases protein expression. Therefore, a principled mRNA design algorithm must optimize both structural stability and codon usage to improve mRNA efficiency. However, due to synonymous codons, the mRNA design space is prohibitively large, e.g., there are $\sim\!10^{632}$ mRNAs for the SARS-CoV-2 Spike protein, which poses insurmountable challenges to previous methods. Here we provide a surprisingly simple solution to this hard problem by reducing it to a classical problem in computational linguistics, where finding the optimal mRNA is akin to finding the most likely sentence among similar sounding alternatives. Our algorithm, named LinearDesign, takes only 11 minutes for the Spike protein, and can jointly optimize stability and codon usage. Experimentally, without chemical modification, our designs substantially improve mRNA half-life and protein expression in vitro, and dramatically increase antibody response by up to 23$\times$ in vivo, compared to the codon-optimized benchmark. Our work enables the exploration of highly stable and efficient designs that are previously unreachable and is a timely tool not only for vaccines but also for mRNA medicine encoding all therapeutic proteins (e.g., monoclonal antibodies and anti-cancer drugs).
PDF Abstract